Covaxin Who Approval Date - Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times
Covaxin manufacturer Bharat Biotech has been submitting data to WHO on a rolling basis submitted additional info at WHOs request on 27 September. As of date India is.

As Indians Who Took Covaxin Await Approval For Travel What S Causing Delay In Who Nod
WHO Chief Scientist Soumya Swaminathan had said earlier that the technical advisory group at WHO will meet on October 26 to consider the Emergency Use.

Covaxin who approval date. It was recommended for use. An AIIMS Professor on Wednesday said that though it is difficult to predict time-frame Bharat Biotechs Covaxin will surely get the WHOs approval. It was announced after.
WHO chief scientist Soumya Swaminathan said on Sunday its technical advisory group would meet on October 26 to consider the listing for Covaxin. Bharat Biotechs Covaxin is yet to be included in the. The latest Status of COVID-19 vaccines within the WHO EULPQ evaluation process guidance document dated 29 September on the WHO website said that the decision date for Bharat Biotechs Covaxin is October 2021.
Bharat Biotech which is currently carrying out the Phase-3 clinical trials for Covaxin informed news agency ANI on Wednesday that it will make the data public during July. The approval process for Covaxin ran into rough weather at WHO blamed on procedural delays by manufacturer Bharat Biotech BB. Though the Indian regulators approved Covaxin in January when the company started its phase 2 trials WHO needs data from phase 3.
The WHOs technical advisory group will meet on October 26 to consider the emergency use listing of Indias Covaxin which is being used in the. The World Health Organisations approval for emergency use of Covaxin can come before the end of the month Dr VK Paul chairman of the National Expert Committee on. 10 Questions the DCGI Needs To Answer About the Two Vaccine Candidates Approved January 2021 Chatterjee said another indication about potential issues with Covaxins phase-3 trial data emerges when comparing Covaxins fortunes with those of vaccines made by Pfizer Moderna and AstraZeneca.
Earlier on October 18 WHO had commented We are aware that many people are waiting for WHOs recommendation for Covaxin to be included in the COVID19 Emergency Use Listing but we cannot cut corners - before recommending a product for emergency use we must evaluate it. WHO experts are currently reviewing this info if it addresses all questions raised WHO assessment will be finalized next week WHO said in a. DNA Web Team.
Pertinently Bharat Biotech had submitted the data required for the WHOs approval for Covaxin on July 9 itself. WHO and an independent group of experts are scheduled to meet next week to carry out the riskbenefit assessment and arrive at a decision on granting EUL to Covaxin. No approval for COVAXIN yet as WHO seeks more data.
The WHO said it began rolling data of the vaccine on 6 July. The first committee has approved the data and the approval for Covaxin will be granted on the basis of what the second committee recommends On October. The World Health Organizations decision on granting emergency use approval for Covaxin has been delayed for a few more days as it has asked for more data from Bharat Biotech sources revealed.
Delay in Covaxins approval WHO awaits crucial info Until Covaxin gets an emergency use authorisation from WHO it cannot get widespread acceptability as a safe and effective vaccine around the world. The emergency use authorisation EUA for Bharat Biotechs COVID-19 vaccine Covaxin has once again been delayed by the World Health. WHO meet next week to discuss Covaxin approval.
WHO said after a meeting on Tuesday that its independent experts had sought additional clarifications from Covaxin maker Bharat Biotech. Oct 19 2021 0805 AM IST. Last week the WHOs expert group had reviewed Covaxin data submitted by Hyderabad-based Bharat Biotech on October 5.
The WHO has approved COVID-19 vaccines by Pfizer-BioNTech AstraZeneca Johnson and Johnson Moderna and Sinopharm till date. The World Health Organizations WHO technical advisory group will meet on October 26 to consider granting Emergency Use Listing EUL to Bharat. WHOs emergency use approval to Covaxin delayed till October 5 Bharat Biotech on Friday said it has submitted all the data to the World Health Organisation WHO.
A WHO panel has postponed granting approval to COVAXIN. Rolling data allows the WHO to start its review right away as information continues to come in to accelerate. WHO decision on emergency use listing for Covaxin likely next week The countrys indigenous Covid-19 vaccine has not yet received emergency use approval from WHO.
Though Bharat Biotech had submitted the data required for the WHOs approval for Covaxin on July 9 the UN health agency was scheduled to take up the proposal for consideration on October 5. The Strategic Advisory Group of Experts on Immunisation SAGE of the WHO will review Covaxin data on October 5 October 6 IST. The World Health Organization WHO has once again postponed granting emergency approval.
Who S Emergency Use Approval To Covaxin Delayed Till October 5 India News India Tv

Who S Approval For Covaxin Bharat Biotech S Pre Submission Meeting On June 23

Good News For Those Who Have Taken Covaxin Who Set To Give Nod To Bharat Biotech S Vaccine Youtube

Covaxin May Soon Get Who Approval As Expert Group Scheduled To Meet Next Month

When Can We Expect Who Approval For Covaxin
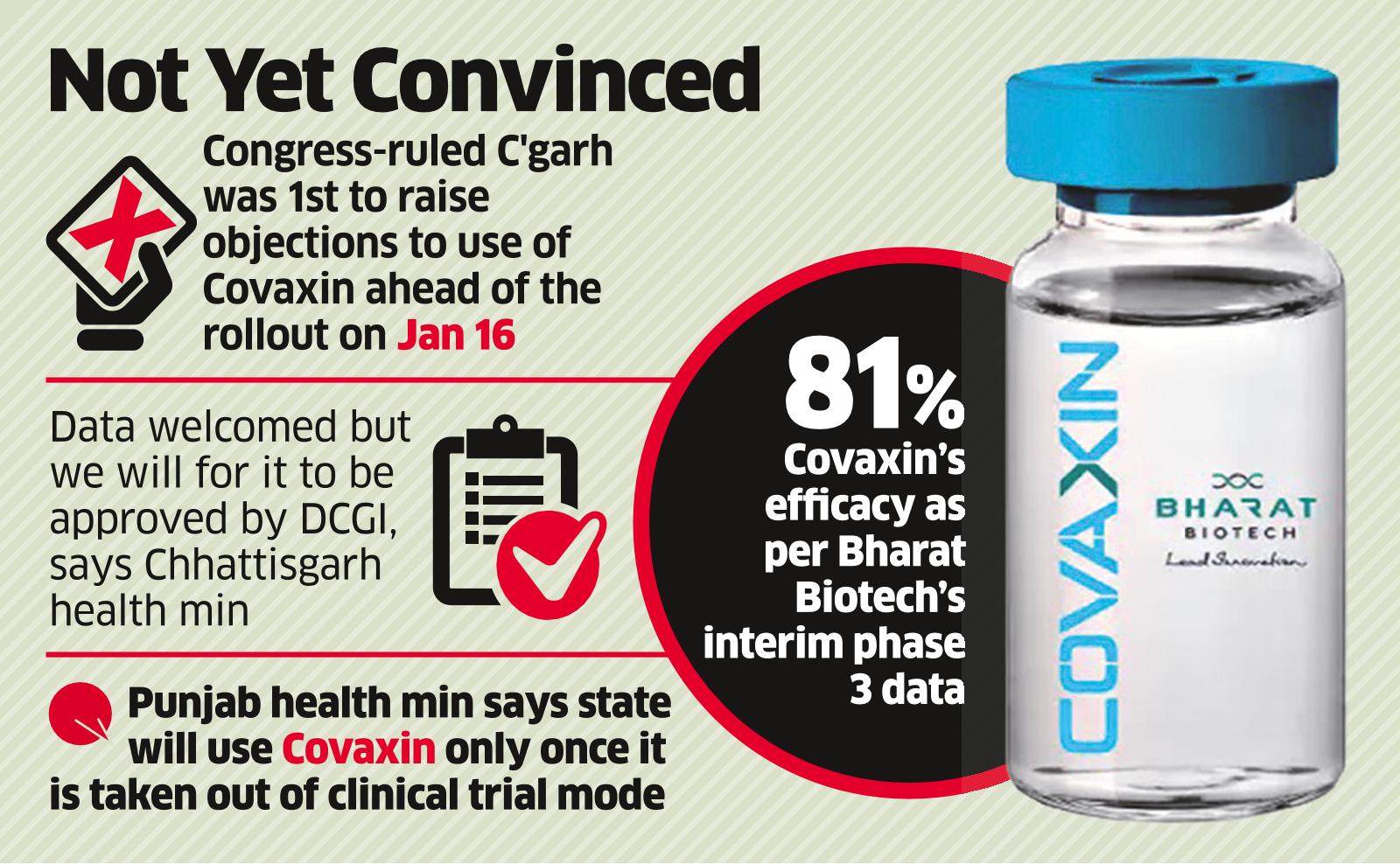
Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times

Covaxin Has Not Got Emergency Use Approval From Who Since 6 Months Know The Process Here
Covaxin Vaccine India News Latest News Top Stories On Covaxin In India
Ocugen Seeks To Sell 100 Mln Indian Vaccine Doses In U S In 2021 Reuters

Covaxin Approval Who S Technical Advisory Group To Decide On 26 October

India S Covaxin Yet To Receive Who Endorsement Govt To Push For Approval The Economic Times Video Et Now

No Approval For Covaxin Yet Who Seeks Additional Clarification To Decide On Eul
Explained What Phase 3 Data Says On Bharat Biotech S Covaxin Vaccine For Covid 19 Explained News The Indian Express

Fda Denies Bharat Bio S Us Partner Emergency Approval For Covaxin Vaccine Business Standard News

Who To Decide On Bharat Biotech S Emergency Approval For Covaxin In October

Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News